X-ray analysis of interfaces
Ionic Liquids and Aqueous Solutions
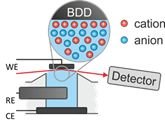
Surface sensitive X-ray scattering experiments can provide an important link between the molecular structure of electrolytes and their ion distribution on free surfaces and buried interfaces. Time-resolved experiments allow us to follow the relaxation dynamics of anions and cations near the electrode after applying an external potential with sub-millisecond resolution.
At the free liquid surface of ILs composed of cations with long aliphatic side chains, we observed surface induced smectic order. This near surface structure exists over a wide temperature range and extends over several tenth of nanometers into the liquid bulk. The lamellar ordering can significantly affect the dynamics of molecules adjacent to the liquid phase boundary by acting as diffusion barrier. Therefore, understanding the formation mechanism of the observed interfacial structures will help to increase the performance in supported ionic liquid phase (SILP) catalysis.
References:
P. Reichert, K.S. Kjær, T.B. van Driel, J. Mars, J.W. Ochsmann, D. Pontoni, M. Deutsch, M.M. Nielsen, and M. Mezger; Molecular Scale Structure and Dynamics at an Ionic Liquid/Electrode Interface. Faraday Disc. 206, 141 (2018).
J. Mars, B. Hou, H. Weiss, H. Li, O. Konovalov, S. Festersen, B.M. Murphy, U. Rütt, M. Bier, and M. Mezger; Surface Induced Smectic Order in Ionic Liquids – An X-Ray Reflectivity Study of [C22C1im]+[NTf2]-. Phys. Chem. Chem. Phys. 19, 2665 (2017).
M. Bier, J. Mars, H. Li, and M. Mezger; Salt-induced microheterogeneities in binary liquid mixtures. Phys. Rev. E. 96, 022603 (2017).
Complex Liquids in Confinement Studied by the X-Ray Surface Forces Apparatus
Soft matter thin films with a thickness of several molecular length scales often exhibit significantly different structural and dynamical properties compared to bulk. Recently, we developed a surface forces apparatus for X-ray experiments (X-SFA) in collaboration with the group of M. Valtiner. Our novel device is optimized for in-situ investigations of liquids in slit pore confinement under compressive forces and lateral shear rates. First experiments on liquid crystals and complex electrolytes confirm the great potential of this experimental approach. Planned and current research projects include questions on the wetting dynamics of surfactants as well as on the structure and dynamics of polymer melts, colloids and self-assembled aggregates in confined geometries.
Reference:
H. Weiss, H.-W. Cheng, J. Mars, H. Li, C. Merola, F.U. Renner, V. Honkimäki, M. Valtiner and M. Mezger; Structure and Dynamics of Confined Liquids – Challenges and Perspectives for the X-Ray Surface Force Apparatus. Langmuir, DOI: 10.1021/acs.langmuir.9b01215 (2019).
Premelting of Ice
The surface melting of ice, discovered by Faraday in 1859, is one of the best-known interface-induced phase transitions. However, the structural and dynamic properties of liquid water within this interfacial premelting layer is still under debate. We study the interfacial premelting in ice/clay nano composites by high energy X-ray diffraction and quasi elastic neutron scattering. Using well defined and characterized ice/mineral composite samples, our work bridges the gap between studies on single crystalline model interfaces and naturally occurring soils and permafrost. Significant differences in the growth of the premelting layer and its mobility are found for the charged vermiculite, uncharged kaolin, and more hydrophobic talc. This indicates that besides confinement, intermolecular interactions between the water molecules and the mineral surfaces play an important role for the water mobility in ice/clay nanocomposites.
References:
H. Li, M. Bier, J. Mars, H. Weiss, A.-C. Dippel, O. Gutowski, V. Honkimäki, and M. Mezger; Interfacial Premelting of Ice in Nano Composite Materials. Phys. Chem. Chem. Phys. 21, 3734 (2019).
Y. Nagata, T. Hama, E. Backus, M. Mezger, D. Bonn, M. Bonn, and G. Sazaki; The Surface of Ice under Equilibrium and Non-Equilibrium Conditions. Acc. Chem. Res. 52, 1006 (2019).
