Unveiling the molecular mechanism of water evaporation
For a water molecule to escape the hydrogen bonds of a liquid and enter the vapor phase requires a well-timed and coordinated dance of at least three molecules, according to newest simulations.
Mainz. Dr. Yuki Nagata and his colleagues from the Max Planck Institute for Polymer Research (MPI-P) in Mainz, Germany have shown that it takes a rare, but very specific sequence of events for a water molecule to evaporate. Evaporation – the transition from liquid to gas – involves the assistance from two other molecules, according to computer simulations. An improved understanding of the evaporation process could help researchers modeling atmospheric processes that are part of climate change. Their results have recently been published in the scientific journal Physical Review Letters.
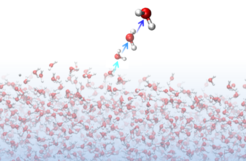
With their results, Yuki Nagata and his colleagues shed new light to the evaporation process. They simulated the water-air interface and watched ‘movies’ of virtual liquid water molecules in silico. From the simulation, they found that a water molecule could only escape the liquid phase if several water molecules at the surface act in unison to eject one of them.
A water molecule is typically tied to three or four other molecules in the liquid through strong hydrogen bonds. At the surface, this number is reduced, and in order to evaporate the molecule must break at least one hydrogen bond. However, this requires substantial energy, and the obvious question is: “How do evaporating water molecules gain sufficient energy to break the strong hydrogen bond?” To answer this, the researchers watched molecules evaporate in their molecular movies, and inspected the evaporating molecules' trajectories. They found that an ejected molecule always gains its kinetic energy through a precise interaction with two other molecules. It always had a violent collision with a fast-moving molecule just prior to leaving the liquid. This fast-moving molecule, further study showed, was interacting strongly with a third molecule, femtoseconds prior to the evaporation process, in a way that was crucial to the evaporation process. As such, the evaporation process can be viewed as a Newton’s cradle, where momentum is transferred to the surface from below, in a well-timed manner, to kick off one water molecule.
The improved understanding of water evaporation at the molecular level can help develop strategies to reduce unwanted evaporation, and is important for modeling atmospheric processes.












