Soft matter under Confinement
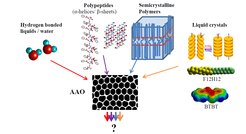
Soft matter is characterized by subtle forces and weak interactions (hydrogen bonds, van der Waals forces, π-π interactions, etc.) that give rise to a hierarchy of organization. By means of dielectric spectroscopy (DS) and complementary techniques we are studying the thermodynamics and dynamics of soft materials under confinement. Confinement here is provided by nanoporous hard templates that provide a two-dimensionally confined space in which self-organization processes such as crystallization, protein secondary structure formation, mesophase formation and phase separation can be altered (Figure 1). Understanding the self-assembly, thermodynamics and dynamics of soft materials under confinement will allow for their rational design as functional devices with tunable mechanical strength, processability, electronic and optical properties.
In recent years there have been concerted efforts in both theory and experiment to understand how, why and when polymers crystallize under confinement. When a polymer melt gets into contact with the opening of a narrow pore the capillary force drags the chains into the pore. An advancement in studying polymer imbibition has been the, so-called, nanofluidic method based on DS (i.e. nano DS). The method provides quantitative information on the imbibition process. In addition, it provides access to length and time scales (from the local segmental, to internal chain modes, to the longest normal mode and to the much slower adsorption process) under non-equilibrium conditions (e.g. during flow) not considered before. In polymers results provide unambiguous evidence for growing interfacial interactions. Taming the strength of interfacial interactions could help towards designing polymer interfaces with controlled physical properties.
In a second area we investigate if and how the ionic conductivity of polymer electrolytes is affected during and after imbibition in nanopores. As an example we used PEO/LiTFSI. By employing nDS we demonstrated that ion conductivity decrease below the bulk value. The reduced ionic conductivity is explained by the adsorption of polymer segments on the pore walls.

In a third area, we investigate how confinement affects the crystallization and dynamics of hydrogen bonded systems, including water. By studying model confining systems we provide new insights into the properties of confined water/alcohols with applications in cryopreservation.
References:

