The secret of the soybean
Mainz researchers are investigating oil bodies in soybeans - new applications in food science
Mainz researchers from the Max Planck Institute for Polymer Research (MPI-P) have used neutron scattering to study small oil bodies in soybeans. These serve the bean when budding and growing as an energy supplier. They are also used in the production of soybean oils. With their investigations, the scientists headed by Prof. Thomas Vilgis (MPI-P, Department of Prof. Kurt Kremer) studied the structure and thus the mechanical stability of these oil bodies. One possible application of their research results is the production of new and innovative foods on a natural basis.
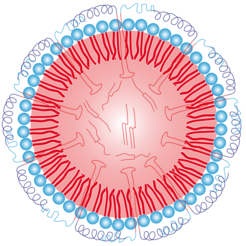
Water and oil do not mix - this is an experience of everyday life. In order to mix water with oil, so-called "emulsifier agents" are needed. One of these is the molecule "Lecitin" (a phospholipid) which is also present in the soya bean. The long-chain molecule has a water-loving as well as a water-repellent (and thus fat-loving) part. The molecule aligns itself around oil droplets and encloses them inside a sphere. The fat-loving part looks inward to the oil. Since the molecule is water-loving to the outside, small oil bodies - consisting of the emulsifier shell and the oily interior - can be dissolved in water. These oil bodies are called Oleosomes.

However, only by the presence of the emulsifier “phospholipid”, it is not possible to explain why oleosomes are stable in the soybean plant for such a long time. "Even small temperature fluctuations and vibrations would actually destroy oleosomes - and the plant would die", says Vilgis.
Therefore, nature creates a stability bonus through special proteins called oleosins. These oleosins penetrate deeply into the oil phase with their elongated and narrow hairpin-shaped middle part like anchors, while two water-loving arms spread protectively over the phospholipids.
In addition, these water-loving arms are electrically charged. This results in an onion-like layered structure for the only a few hundred nanometers large oleosomes which consists of the ends of the protein arms reaching into the water, the underlying phospholipids, the protein anchors penetrating into the oil and the oil core itself.
For the scientists of the "soft matter food physics" group in Mainz, these naturally occurring nanoparticles have long been the focus of research, but the exact structure of the oleosome was previously unknown. A deeper insight was possible by an accurate analysis of Small Angle Neutron Scattering-experiments. For this purpose, neutrons were shot at the nanoparticles at the research reactors in Grenoble and Oxford, and conclusions about the structure of the particles were drawn from their deflection.
This is made possible by contrast variation methods with mixtures of different concentrations of "heavy water" (whose hydrogen atoms have been replaced by deuterium) and "normal water". The neutrons are deflected completely differently by deuterium and hydrogen, what can physically be described with the so-called “scattering length”. These lengths of the two sorts of water even have a different sign. Thus, similar to the selection of corresponding indices of refraction in optics, different layers of the oleosome can be selectively faded out and in for the neutrons. From the patterns of the scattered neutrons, the structure and size of the oil bodies can be determined.
The researchers were able to determine the diameter of the oleosome to 278 nanometers. The outer layer, the protein shell protruding into the water, is 9 nanometers thicker than previously thought. The reason for this are the positive electric charges that are present on it: because of their repulsion, only the way out in the aqueous environment of the cell and thus away from the oleosome remains. The temperature stability of the oil bodies up to 90 ° C could be directly verified by the neutron scattering measurements.
The precise knowledge of the structure of the soybean nanoparticles results in a whole series of targeted applications. Such natural nanoparticles can be used to selectively place and transport nutrients that are soluble in water and fat. While oil-soluble nutrients (e.g., vitamins) can be diffused into the interior of the particles, water-soluble substances can adhere to their surface. This is made possible by the electrically charged oleosines, whose charge can be controlled via the pH value. The oleosomes are positive in a acidic environment, negatively charged in a basic environment. This allows the nanoparticles to be "encapsulated" in a variety of ways with biopolymers of opposing charge. This has been for example performed in the past already with pectin - a known sugar and gelling agent. Again, the layer thickness of the pectin could be measured with neutron scattering. This makes new forms of oleosome-based plant foods possible. Furthermore, the findings are not limited to soybeans, they can also be extended to the oleosomes of other oilseeds (hazelnuts, flax seeds, ...). New approaches, e.g. for the geriatric nutrition can also be realized.
About Thomas Vilgis
Thomas Vilgis was born in 1955 in Oberkochen. After studying physics and mathematics in Ulm and the following doctorate, he moved to the Cavendish Laboratory in Cambridge. In 1990 he habilitated at the University of Mainz, where he was appointed professor in 1996. Since 1985 he has been research group leader at the Max Planck Institute for Polymer Research in Mainz.
In addition to his research, Thomas Vilgis is known for his numerous book publications in the field of physical and chemical aspects of food and a guest on radio and television shows. His "Journal Culinaire", where he is the publisher, was recently named "Best of the World - 2nd Place" at the "World Cookbook Award".













